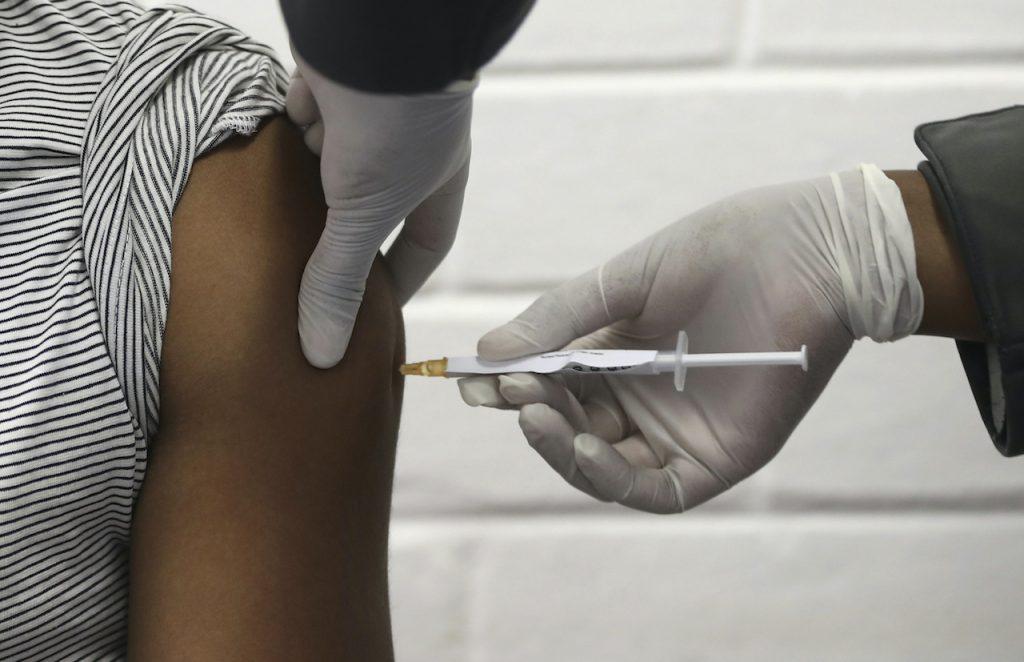
More European nations pause use of Oxford-AstraZeneca vaccine
This is threatening to have deadly consequences as major European nations all have current high rates of infection.
Just In
A number of countries have suspended use of the Oxford-AstraZeneca vaccine as a precaution following reports that some people have suffered blood clots after the jab.
Their decisions have been made on the basis of the precautionary principle – a well-established approach in science and medicine that stresses the need to pause and review when evidence is uncertain, says the BBC.
But in a fast-moving pandemic, when each decision can have major consequences, it is an approach which can sometimes do more harm than good.
The data supplied by AstraZeneca shows there have been 37 reports of blood clots among the 17 million people across Europe who have been given the vaccine.
The World Health Organization (WHO) and UK drugs regulators have all said there is no evidence of a link.
The European Medicines Agency, which is looking into the reports, has suggested the vaccine should continue to be used given the risk Covid presents to health.
Professor Adam Finn, a member of WHO’s working group on Covid vaccines, says stopping rollout in this way is “highly undesirable” and could undermine confidence in the vaccine, costing lives in the long-term.
This is not the first time countries in Europe have exercised caution about the AstraZeneca vaccine.
The precautionary principle was adopted by Germany, France and others when they did not initially recommend use of the vaccine for the over-65s.
The over 65s decision has now been reversed, but the impact is still being felt as Germany and France have supplies of the vaccine going to waste, with both countries having used fewer than half their supplies of the AstraZeneca jab so far.
This is threatening to have deadly consequences. France, Germany and the other major European nations all have higher rates of infection than the UK and face the prospect of things getting worse before they get better.
The UK used the vaccine while other countries dithered and within months was reporting “spectacular” results in reduced levels of serious illness in the over-80s.
With cases rising rapidly at the time, the UK was clear the benefit of maximising available vaccine supplies to provide some protection to more people was the right step even if the trial evidence did not directly support it.
Analysts have also suggested that EU slowness in getting started with their vaccine programme has hamstrung the whole process.
And political motives for EU countries looking for ways to call the British vaccine into doubt have not been ruled out and might have something to do with lingering continental animosity over Brexit.
Professor David Spiegelhalter, an expert in understanding risk at Cambridge University, says it shows that sometimes people have to look beyond the precautionary principle and be bold in their decision-making.
“The precautionary principle favours inaction as a way of reducing risk. But the problem is that these are not normal times and inaction can be more risky than action,” he said.
“Sometimes it can be harmful to wait for certainty. Not vaccinating people will costs lives.”
Subscribe to our newsletter
To be updated with all the latest news and analyses daily.